For older people and frail people, the long-term benefit of medicines reduces and the potential for harm from adverse effects increases. When the benefit–risk balance changes in this way, medicine review and optimisation are important to simplify the therapeutic regimen, reduce inappropriate medicines and minimise risks. In this article, pharmacist prescriber Linda Bryant uses two case studies to illustrate important considerations during medicine reviews
COPD: Prevent, treat and comfort
COPD: Prevent, treat and comfort

Respiratory physician Lutz Beckert considers chronic obstructive pulmonary disease management, including the prevention of COPD, the importance of smoking cessation and pulmonary rehabilitation, and the lifesaving potential of addressing treatable traits. He also discusses the logic of inhaler therapy, moving from single therapy to dual and triple therapy when indicated, as well as other aspects of management
This How to Treat has been endorsed by the RNZCGP and has been approved for up to 2 credits for continuing professional development purposes (2 credits per learning hour). To claim your CPD credits, complete the assessment at the end of this page, then log in to your Te Whanake dashboard and record this activity under the appropriate learning category.
Other healthcare providers may also find that reading this article and reflecting on their learning can count as a professional development activity with their own professional association.
At the end of this course, you should be able to:
-
Discuss modifiable risk factors for COPD
-
Describe traits that confer worse outcomes for patients with COPD, and their treatment
-
Explain how COPD is diagnosed and how severity of disease is assessed
-
Discuss the management of COPD, including inhaler therapy
The subtitle of this article does not flow as nicely as the wisdom of Edward Livingston Trudeau, the US physician who founded the late 19th century tuberculosis sanatorium: “to cure sometimes, to relieve often, to comfort always.”
The need for human connection holds true in general practice and in outpatient clinics, with a need for care, comfort and kinship. We have made advances in non-invasive ventilation, long-term oxygen therapy and lung transplantation; however, this is largely irrelevant for the patients with chronic obstructive pulmonary disease who attend our clinics with lips pursed, chests heaving and hearts racing. So, what do we have to offer?
I couldn’t use Dr Trudeau’s aphorism because we cannot cure COPD. The evidence surrounding the prevention of COPD has become better, our treatment options – using the treatable traits approach – have become clearer, and our role as healthcare professionals to comfort our patients is as important today as it was 100 years ago. For most of our patients with COPD, primary care is the ideal place to prevent, treat and comfort.
Young lungs
Primary care is ideally positioned to look after young lungs. That, of course, includes care for pregnant people by facilitating healthcare access, supporting smoking cessation and encouraging breastfeeding.
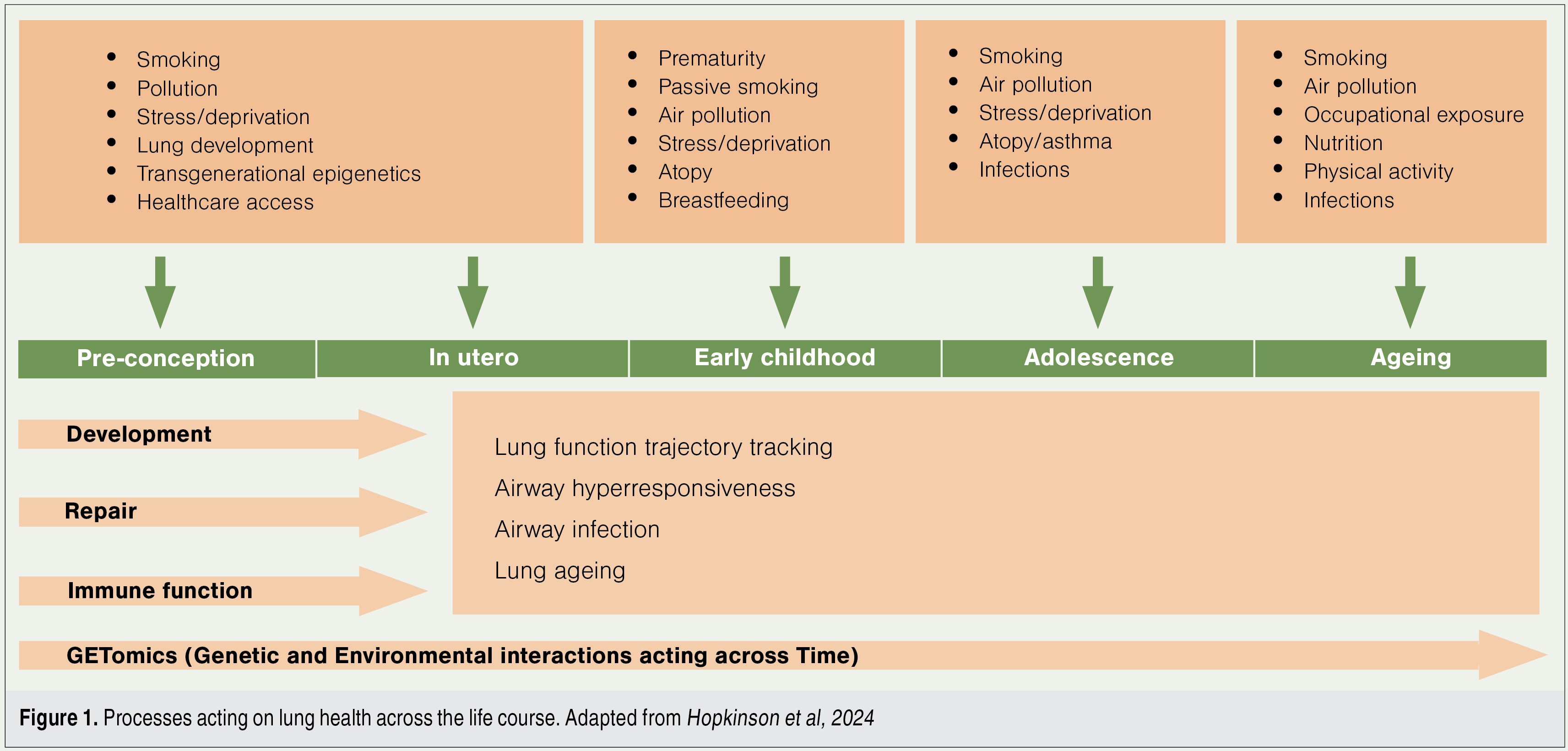
We now have the benefit of several longitudinal studies following participants into their seventh decade of life, and more than 40 European research groups have come together to form the collaboration of Chronic Airway Diseases Early Stratification (CADSET).1
These studies have shown that the first 1000 days of life (ie, from conception until the second birthday) underpin lung health, harbour the origins for development of lung disease, and are crucial for lifelong lung health (Figure 1).2
One positive flow-on effect of our tobacco control measures is a reduction in preterm births. Prematurity has been associated with early emphysema, altered immune function, and telomere shortening, a marker of premature ageing. Lower respiratory tract infection in the first two years of life is associated with an almost doubling of premature death in adult life.
In 2025, the monoclonal antibody palivizumab is funded for the respiratory syncytial virus (RSV) season to prevent severe illness in high-risk children, and we are on the threshold of having antenatal vaccination available. Nirsevimab (the successor to palivizumab) and the vaccine Abrysvo are not yet approved in Aotearoa New Zealand but have been approved in Australia. There is hope these interventions will have the short-term effect of reducing the mortality and morbidity of severe RSV infections. They may also reduce the development of asthma and severe airway disease in the long term.
Smoking increases risk of COPD 2.8× Smoking and exposure to vapours, gases, dusts and fumes increases risk 6.2×
Adult lungs
It is not only the paediatricians who are optimistic we can prevent COPD. In 2022, a landmark article aimed to “set the course to eliminate the disease by challenging accepted dogma and generating debate”. The Lancet Commission statement made the point that the stepwise approach of incrementally increasing our knowledge and treatment options has failed patients. It called for a wider understanding of risk factors, highlighted the devastating effects of global poverty, and articulated pre-emptive measures to avoid future cases of COPD.3
The authors also challenged us to rethink the diagnostic criteria for COPD to allow earlier diagnosis, such that early pathological changes amenable to reversal are identified.3 This may require new lung function tests, such as forced oscillometry, or use of artificial intelligence during lung cancer screening programmes.
New Zealand has not yet made a decision on lung cancer screening; however, our Australian colleagues will start lung cancer screening this year. A good general practice database including smoking data may become the gatekeeper for screening eligibility – no data on smoking, no CT scan.
On a global scale, COPD is a frequent sequela of tuberculosis and is often associated with HIV; both diseases have reliable treatments. In addition, passive smoking causes lung damage, vaping promotes airway inflammation, and smoking cannabis increases coughing, sputum production and wheezing. Research on the effects of cannabis on lung disease is lacking. Nonetheless, cannabis is becoming legalised in many countries, one-quarter of the world’s population have tried it, and about 17 per cent of cannabis smokers also use tobacco cigarettes. Currently, cannabis is the world’s most commonly used illicit drug.
Occupational exposure
A third avenue for preventing COPD is to reduce environmental exposure. It is estimated that through the effects of air pollution, approximately 90 per cent of us are exposed to lung irritants. Worldwide, women carry the largest burden of airway disease through exposures via unfluted cooking and associated burning of wood, crop waste or coal.
In developed countries, it is estimated 14 per cent of COPD cases can be attributed to exposure to vapours, gases, dusts and fumes – occupational COPD may be the most under-recognised occupational lung disease. A North American study suggested that exposure to vapours, gases, dusts and fumes increases the risk of COPD 1.4-fold, smoking alone increases the risk 2.8-fold, and exposure to vapours, gases, dusts and fumes as well as smoking increases the risk 6.2-fold.4
The most comprehensive summary is in “The Lancet Commission on pollution and health” and its updates.5
Pulmonary rehabilitation
Doing any type of exercise is almost counterintuitive when you have a chronic illness, particularly a chronic illness that already causes shortness of breath. And yet, more than half of all participants in a Better Breathing Programme will report an improved walk distance, reduced symptoms and increased quality of life. It is the intervention with the lowest number needed to treat to gain benefit, far outperforming any pharmaceutical intervention.
Naturally, we ought to prescribe inhalers that relieve patients’ breathlessness (discussed later); however, this should be in combination with a referral to pulmonary rehabilitation. Without exercise, we deprive our patients of the most powerful intervention currently available.
Furthermore, no article on COPD can omit mention of smoking cessation and pulmonary rehabilitation. Our messaging to the Ministry of Health to fund both spirometry and pulmonary rehabilitation is clear and unambiguous. In Australia, both have become part of the Chronic Obstructive Pulmonary Disease Clinical Care Standard.6
Treatable traits
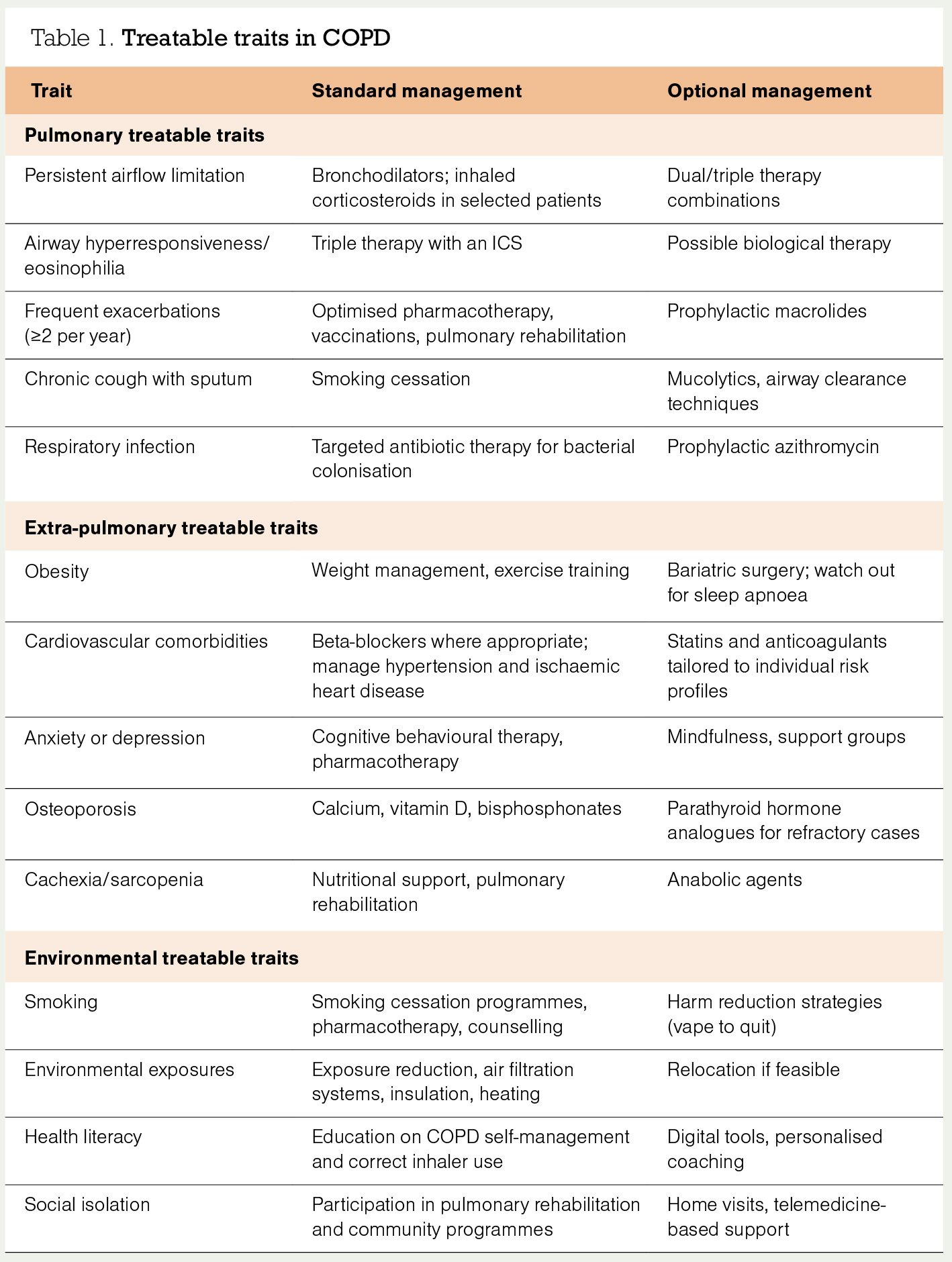
One could argue that specialist clinics have learnt the art of the possible from colleagues in primary care. Treatable traits is a model of care designed to address the heterogeneity of patients with COPD. It involves a multidimensional assessment and deconstructs airway disease into traits.
A treatable trait can be characterised, targeted with treatment, and becomes part of the management plan. Using treatable traits enables an individualised approach to treatment, clear definition of treatment outcomes, and appropriate referral to other healthcare providers. Some general practices engage a healthcare coordinator or health improvement practitioner to synchronise and coordinate the multidimensional, multidisciplinary personalised care.
Table 1 lists some of the frequent COPD treatable traits, their biomarkers, and common treatment suggestions.
Cardiac comorbidities
The presence of airflow obstruction doubles the risk of sudden cardiac death! So, the third aspect to highlight under treatment is the somewhat neglected treatment of cardiac comorbidities in patients with COPD.
The evidence for cardiac involvement is overwhelming – in patients with COPD, we also find coronary artery calcification in 80 per cent, heart failure in up to 42 per cent, and ischaemic heart disease in up to 20 per cent. The heart and lungs have a close anatomical connection, intimate physiological coupling and share many risk factors for disease.
In addition to the shared risk factors and synergistic impact, treatment for COPD may worsen cardiac outcomes. For example, treatment of airway disease with azithromycin increases the risk of cardiac death in patients at highest risk of cardiovascular events. The risk of bronchoconstriction with cardioselective beta-blockers is almost inconsequential; however, many patients with COPD do not receive beta-blockers despite documented heart failure or ischaemic heart disease.
Improving outcomes in patients with both COPD and cardiovascular disease is a major research focus for this decade,7 and New Zealand Doctor Rata Aotearoa may pick this up in a future issue.
Spirometry to identify COPD
Finally, we come to the question at the top of most patients’ priority list: “Can you make me less short of breath?” By the time we address this question, we will have communicated that we cannot undo damage to the lungs, that patients need to have agency to manage their breathlessness, and that together, we will work through the treatable traits discovered during the clinical interview.
Patients referred to my own practice have normally been so well treated for their airway disease that I frequently endorse the treatment by the primary care team and spend time addressing treatable traits. However, population-based studies suggest we miss making the diagnosis of COPD in up to 80 per cent of cases, so I never see these patients in my hospital-based outpatient clinic.
Crucial for making a diagnosis of airway disease is access to spirometry. Access to simple spirometry testing is patchy throughout New Zealand. In October 2024, the Asthma and Respiratory Foundation NZ held a Spirometry Think Tank workshop and drafted a briefing, to visualise the disparities, articulate an action plan and engage with the Ministry of Health (tinyurl.com/Spirometry-TT).
It may be helpful that the Australian Commission on Safety and Quality in Health Care recently set a Clinical Care Standard for COPD, with the first quality statement pertaining to access to high-quality spirometry for any person over the age of 35 with risk factors and symptoms.6 Since access to spirometry has made it into this standard of care, we hope to make the case for adequate funding for spirometry in New Zealand.
The diagnosis of COPD is based on spirometry – ie, a forced expiratory volume in one second (FEV1) to forced vital capacity (FVC) ratio less than the lower limit of normal, or <0.7 as defined by the Global Initiative for Chronic Obstructive Pulmonary Disease.8 However, the severity assessment of COPD, which drives medication prescribing, is based on history of exacerbations and the magnitude of respiratory symptoms.
Exacerbations of COPD are an important outcome marker. Some researchers suggest using the term “lung attacks” to underline their significance to lung health, reduction of health status and worsening survival. Exacerbations are defined as episodes of an acute worsening of respiratory symptoms associated with local and systemic inflammation.8 The best predictor of further exacerbations is a history of previous exacerbations. Preventing exacerbations is an important treatment goal.
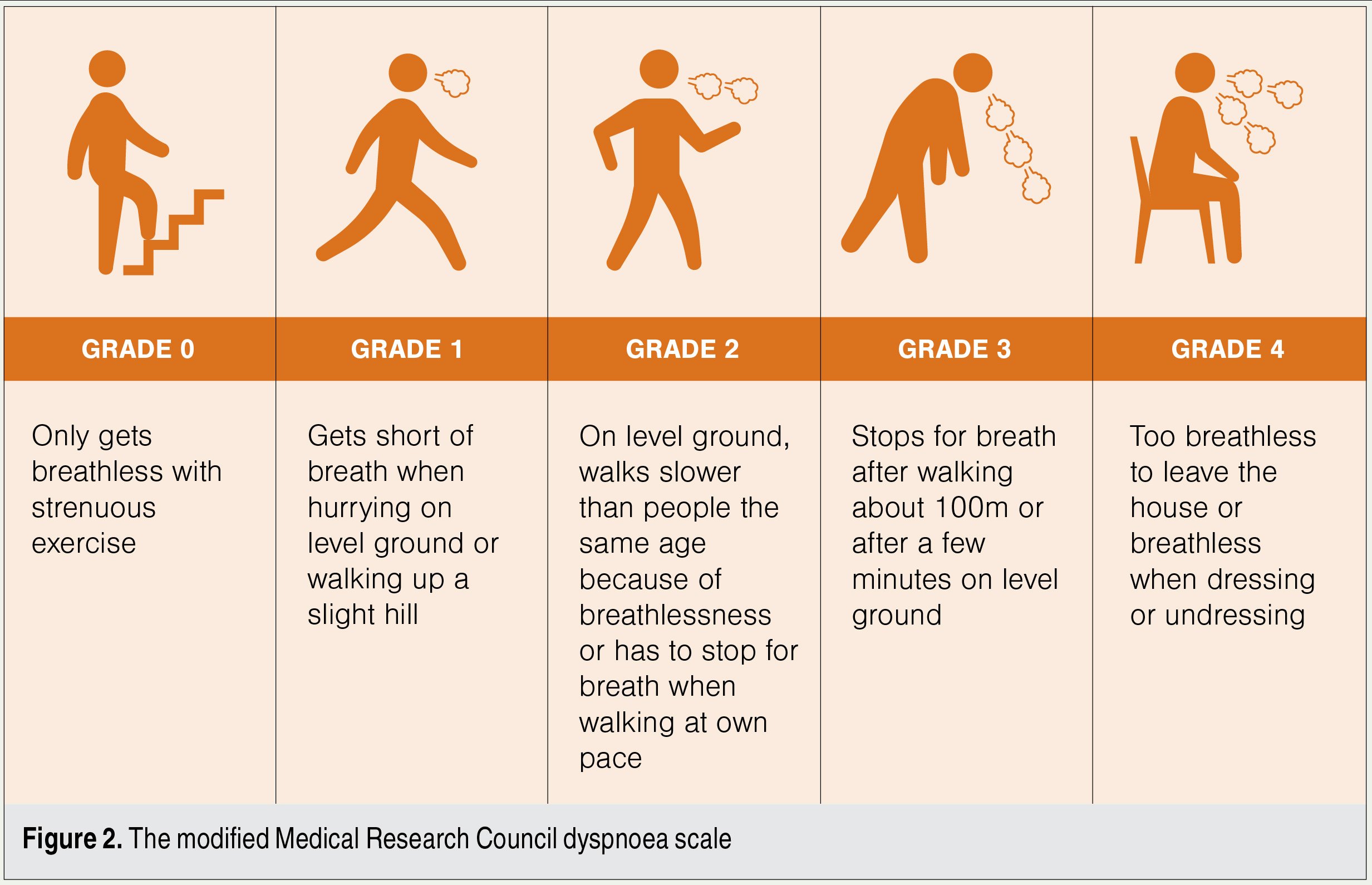
Respiratory symptoms are best assessed with the modified Medical Research Council (mMRC) dyspnoea scale (Figure 2). It is well-validated, simple and correlates well with future mortality risk.
One of the best validated short questionnaires is the eight-item COPD Assessment Test (CAT), which considers symptoms beyond dyspnoea. Not only is this an easy-to-use clinical tool but it is also integrated into the Pharmac access criteria for triple-inhaler therapy, where a score greater than 10 is the cut-off for Special Authority funding.
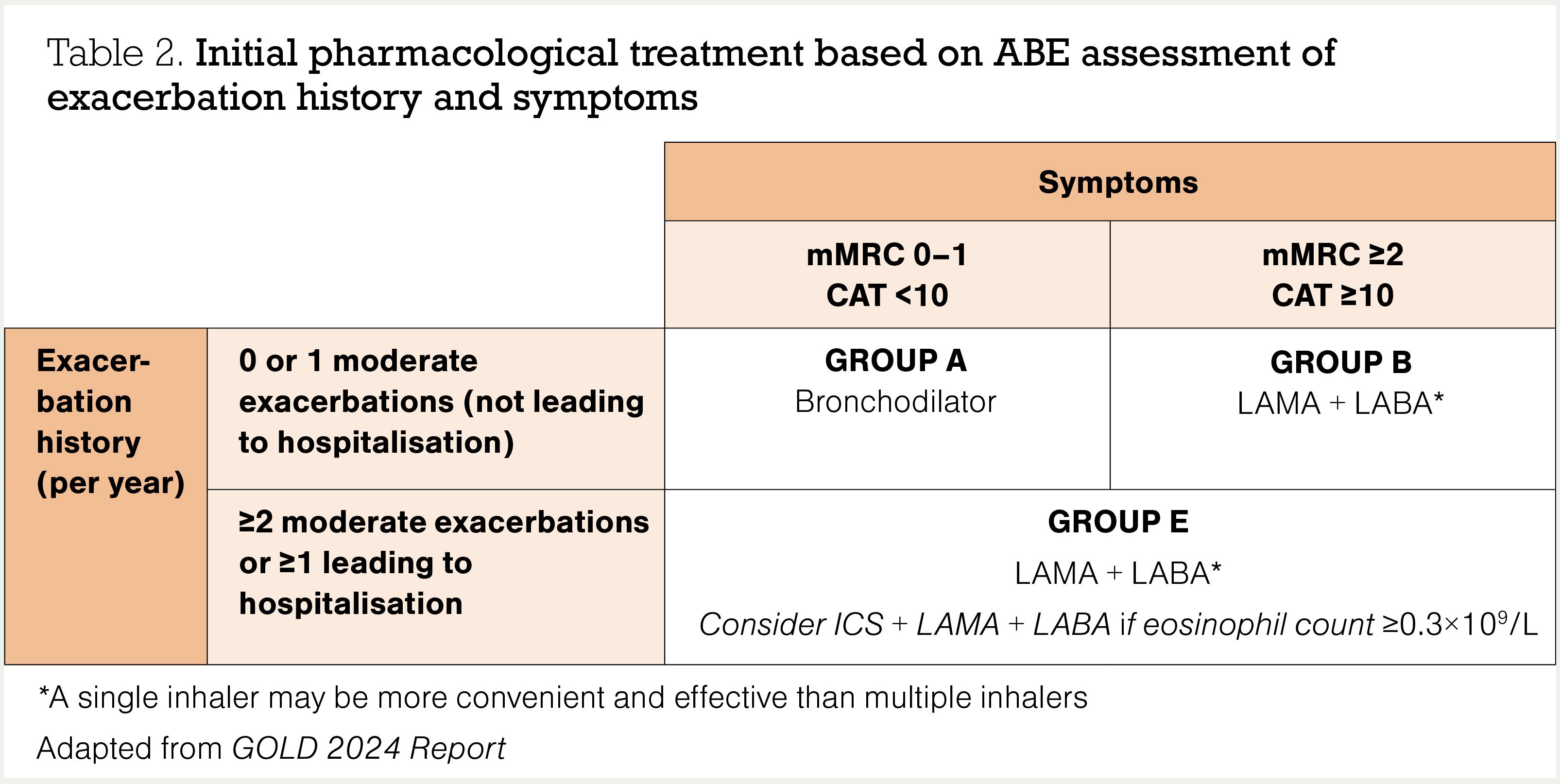
So, we use spirometry to diagnose airway disease, and we use a combination of exacerbation history and symptoms to assess the severity of COPD, explore the prognosis and guide pharmacological management (Table 2).8
The routine use of ICS + LABA in COPD is not recommended
Inhaler therapy
Group A
Depending on the cohort studied, about half of all patients with COPD are in pharmaceutical treatment group A. All patients in this group should be offered a trial of bronchodilator therapy. This can be a short-acting beta2 agonist (SABA) such as salbutamol, or a short-acting muscarinic antagonist (SAMA) such as ipratropium.
I normally commence these on an as-needed basis to reduce the risk of toxicity, which is dose related. Common adverse effects of SABAs are tremor, tachycardia, arrythmias and muscle cramps. Common adverse effects of SAMAs include dry mouth, blurred vision, constipation, urinary retention and postural hypotension.
If patients gain benefit from bronchodilator therapy and take them on a regular basis, I tend to swap them to either a long-acting beta2 agonist (LABA) such as formoterol, indacaterol or salmeterol, or a long-acting muscarinic antagonist (LAMA) such as glycopyrronium (Seebri Breezhaler), tiotropium (Spiriva Respimat) or umeclidinium (Incruse Ellipta).
Group B
A little more than one-quarter of all patients tend to be in group B. Most of them will be managed in primary care as – per definition – they have one or no exacerbations per year. As mentioned above, these patients are very likely to have comorbidities or other treatable traits, which will impact on their prognosis, determine treatment options and call for control of symptoms.
These patients are normally offered treatment with LAMA + LABA combination therapy. We are fortunate in New Zealand to have three LAMA + LABA combination inhalers available – glycopyrronium + indacaterol (Ultibro Breezhaler), tiotropium + olodaterol (Spiolto Respimat) and umeclidinium + vilanterol (Anoro Ellipta).
Group E
Think of E for “exacerbations” or “eosinophilia”. A little less than one-quarter of all patients with COPD have exacerbations more than once a year. These also tend to be the small fraction of patients who are seen in secondary care, often in the context of exacerbations.
The following principles of treatment apply to patients in group E:
- Initial management is still with a LAMA + LABA combination inhaler.
- The routine use of inhaled corticosteroid (ICS) + LABA in COPD is not recommended.
- If an ICS is indicated, triple therapy with ICS + LAMA + LABA in a single inhaler should be prescribed – budesonide + glycopyrronium + formoterol (Breztri Aerosphere) or fluticasone furoate + umeclidinium + vilanterol (Trelegy Ellipta) are currently funded (see panel).
- The best evidence for triple therapy is in the group of patients with proven COPD who have had a blood eosinophil count ≥0.3×109/L in the previous 12 months.
- Pharmac also funds single-inhaler triple therapy for patients with established COPD who have a CAT score >10, or have had exacerbations in the previous 12 months, or are already on triple therapy using multiple inhalers.
Patient has a diagnosis of COPD (confirmed by spirometry or spirometry has been attempted and technically acceptable results are not possible)
AND
is currently receiving an ICS + LABA or LAMA + LABA (or is currently receiving multiple-inhaler triple therapy and met at least one of the clinical criteria below prior to commencing multiple-inhaler triple therapy)
AND
- has a CAT score >10 OR
- has had two or more exacerbations in the previous 12 months OR
- has had one exacerbation requiring hospitalisation in the previous 12 months OR
- has had an eosinophil count ≥0.3×109/L in the previous 12 months.
Adapted from Pharmac Special Authority forms SA2326 and SA2421
Other considerations
Here are three more comments about the choice of inhaler therapy:
- We have left the term asthma-COPD overlap syndrome behind. If patients also have asthma, an ICS is a mandatory part of treatment.
- As part of our regular meetings with patients, we should review their symptom control, assess inhaler technique and adherence, and adjust the treatment accordingly (including down-titration because of side effects and up-titration because of suboptimal symptom control).
- Propellants have an impact on global warming. Good reviews are available on this topic. As a rule of a thumb, one salbutamol metered dose inhaler has a similar environmental impact to driving 150km. Dry powder and fine mist inhalers have much lower carbon footprints.9
Vaccination
Patients with COPD benefit from vaccination, and recommendations could be structured into funded and non-funded vaccines:
- Funded vaccines – patients with COPD should be offered the yearly influenza vaccine. They could also consider a yearly update of COVID-19 vaccination.
- Recommended but non-funded vaccines – consider a pertussis (whooping cough) update; the 13-valent pneumococcal conjugate vaccine (PCV13) followed by the 23-valent pneumococcal polysaccharide vaccine (23PPV) at least eight weeks later (a maximum of three doses of 23PPV can be given in a lifetime, a minimum of five years apart); and the new RSV vaccine (Arexvy) for adults aged 60 and older.10
Oxygen therapy
Many patients harbour the hope that oxygen will improve their breathlessness. Sadly, that is not the case, and studies have consistently shown oxygen therapy does not reduce breathlessness, hospital admission or functional impairment. In the correct patient, it may improve survival – frequently, these happen to be the same patients who are reluctant to use oxygen. Also, patients with low-to-normal oxygen saturations (eg, <92 per cent) at rest may need supplementary oxygen during air travel.
The research is in flux. The studies demonstrating the survival benefits of oxygen are about 50 years old. It is possible patients gain more from ventilatory support at home than from oxygen therapy. Currently, respiratory services are mainly funded to provide acute non-invasive ventilation in patients presenting with hypercapnic respiratory failure in an inpatient setting. However, in the future, we may have an evidence base for providing ventilatory support at home to improve hospitalisation-free survival – something to keep an open mind for in the next update on COPD management.
Palliative care
We need to briefly consider the different narratives of our patients. Patients with (lung) cancer normally have clear time frames, a predictable illness trajectory and a well-defined prognosis. Patients often present their story as a “quest” (I had this cancer, and I am beating it) or a “mission” (I ended up with cancer, and I will work to ensure this does not happen to others).
This is very different to patients with COPD. It is hard to define the “beginning” of COPD. Patients frequently recount being a “wheezy child”, have had symptoms for many years, and cannot really recall acquiring the diagnostic label of COPD. The narrative of a COPD illness is frequently unstructured or chaotic, with not even a logical sequence of events (this happened, that was stressful, and this treatment didn’t work). It is difficult for doctors and patients to determine onset of COPD and often equally as difficult to define when the final phase of the COPD illness trajectory commences.
If we do not attempt to define the illness trajectory, identify the final two years of life, and have a discussion about shared goals of care, then patients miss out on appropriate care in this phase of their lives. However, in the latter stages of a patient’s life, physiological data becomes less helpful – all patients have severe COPD, all are receiving maximal therapy, and all have frequent exacerbations.
Local and international audits provide some guidance on prognosis following a period of non-invasive ventilation during an exacerbation. As a rule of thumb, about 50 per cent of patients die within two years following the need for non-invasive ventilation. Another way to identify patients in their final phase of life is to step back and take a “helicopter” view of their life: do they still have hobbies, do they need support with personal care, have they rearranged their house, or do they have panic attacks?11
The acknowledgement that patients are in their final phase of life (ie, may have less than two years to live) often reduces guilt and frustration in patients, whānau and healthcare professionals.
Treatments focused on reducing symptoms can include the use of a fan, nutritional support and pharmaceutical treatment of anxiety and depression. A comprehensive Lancet review presents options for palliative care and management of troublesome symptoms.12 At times, specialist palliative care may be able to assist patients in this phase of life.
COPD has a low disease status, is plagued by therapeutic nihilism, and is compounded by social factors…We can’t change it all, but we can share with our patients that we are aware of the structural limitations they face
Thank you for making it to the end of this short review on the management of COPD. In New Zealand, we have the opportunity to collaborate to improve lung health. Here, I conclude by briefly exploring structural issues and our attitudes towards lungs in the community.
In 2024, the Norwegian sociologist Johan Galtung died at the age of 93. In 1969, he coined the term “structural violence”, making the case that in addition to obvious physical harm, violence can also be built into systems to perpetrate adverse outcomes for patients. In April 2024, authors from the Imperial College London published a concise clinical review on COPD as a manifestation of structural violence.13 A 2024 study showed New Zealand patients essentially share similar experiences, leading to health inequities.14
Working in primary care, don’t you sometimes have the feeling that the system works against patients when you try to access spirometry, psychological care or pulmonary rehabilitation? COPD has a low disease status, is plagued by therapeutic nihilism, and is compounded by social factors such as limited transport options, social isolation and poor housing. We can’t change it all, but we can share with our patients that we are aware of the structural limitations they face in addition to their personal symptoms and experiences.
Furthermore, advocacy to improve health policy and health systems is part of our professional framework defined by the professional colleges: “Physicians deliver and advocate for the best health outcomes for all patients and populations.”15
Some of our respiratory colleagues have started an uplifting initiative where they share stories, anecdotes and hints on how to communicate positively about lung health. Hint: we can probably learn a lot from the cardiologists. I recommend reading “I love you with all my lungs: a viewpoint on communicating effectively and positively about lung health”, a short three-page article with powerful figures.16
Hopefully, I have made the case in this article that COPD can be prevented, symptoms actively treated, and quality of life improved. Thanks for your keenness, lungly yours!
“These inhalers don’t really work”, mumbles Henare as he unpacks a colourful array on your desk. Henare does not attend often; he does not like being “told off” for smoking, and the practice opening times interfere with his work time as a linesman.
Recently, your practice had engaged the services of a health improvement practitioner, which has improved access to care for Henare. On her suggestion, he had received the influenza vaccine and COVID19 update, and through the process of whakawhanaungatanga, she discovered Henare and his whānau are heavily involved with kapa haka.
Your notes tell you Henare is Māori and that he had spirometry during an admission to hospital about eight months ago; his FEV1 was 59 per cent of predicted without reversibility. He has had two hospital admissions in the last two years; the first was COVID19 related. He has a family history of ischaemic heart disease. Five years ago, he had a road traffic accident and stopped smoking shortly afterwards.
Henare explains that he is becoming short of breath with associated chest tightness when walking up stairs. He is concerned about his breathing. He is a new grandfather, has developed a strong bond with his grandson and wishes to be part of his life.
You congratulate him on his smoking cessation, encourage the physical activity associated with kapa haka and explore cardiac risk factors. You treat his hyperlipidaemia, prescribe a cardioselective betablocker and glyceryl trinitrate, arrange for an electrocardiogram, and make a nonurgent referral to cardiology.
You change his COPD treatment to singleinhaler triple therapy (COPD proven by spirometry, currently on ICS + LABA, and hospital admission in the last 12 months). You explain that you expect the single inhaler to reduce his risk of hospitalisation and improve his walking/kapa haka performance by 20 per cent.
You ask the HIP to stay in touch with Henare so as not to interfere with his work hours, and you schedule a review to discuss nonfunded but recommended vaccinations for whooping cough, pneumococcal disease and RSV.
Before then, you will investigate vaccine funding options to reduce the cost barrier for Henare. Finally, you make a note to enquire about his kapa haka and engagement with his grandson once he has more energy.
Lutz Beckert is a respiratory physician at Health New Zealand Te Whatu Ora Waitaha Canterbury and professor of medicine at the University of Otago, Christchurch
This 10-question multiple-choice assessment is designed to demonstrate that the provided educational reading has been effective in allowing you to meet the learning objectives of this course. Write down your answers to these questions.
1. Select three modifiable risk factors for chronic obstructive pulmonary disease?
a. Childhood respiratory infections
b. Exposure to air pollution
c. Family history of asthma/COPD
d. Reaching developmental milestones
e. Smoking
2. What is the most effective intervention for COPD?
a. Bronchodilators
b. Oxygen therapy
c. Pulmonary rehabilitation
d. Triple therapy with an inhaled corticosteroid
3. Which model of care used in COPD management involves a multidimensional assessment to identify patient characteristics that, when targeted with treatment, improve outcomes and quality of life?
a. Preventable traits
b. Stepwise therapy
c. Targeted treatment
d. Treatable traits
4. Name one treatable trait in COPD. How would you manage it?
5. What is spirometry used for in COPD management?
a. Diagnosis
b. Pharmacotherapy guidance
c. Prognostication
d. Severity assessment
6. Which two statements regarding exacerbations in COPD are correct?
a. A patient who has experienced one exacerbation leading to hospitalisation in the last year is in pharmaceutical treatment group B
b. Exacerbations are defined as episodes of an acute worsening of respiratory symptoms associated with local and systemic inflammation
c. History of exacerbations and the magnitude of respiratory symptoms are used to guide medication prescribing
d. The best predictor of exacerbations is the blood eosinophil count
7. Brian says he has to stop to catch his breath every few minutes when walking, even when just walking through the mall. What grade is he on the modified Medical Research Council dyspnoea scale?
a. Grade 1
b. Grade 2
c. Grade 3
d. Grade 4
8. Patients in COPD pharmaceutical treatment groups A and E are normally initially managed with which of the following inhaler therapies?
a. ICS + LABA
b. ICS + LAMA + LABA
c. LAMA + LABA
d. SABA or SAMA
9. Which two of the following are listed in the current Pharmac Special Authority criteria for single-inhaler triple therapy for patients with COPD (currently receiving ICS + LABA or LAMA + LABA or multiple-inhaler triple therapy)?
a. Has a COPD Assessment Test score <10
b. Has an mMRC score ≥2
c. Has had an eosinophil count ≥0.3×109/L in the previous 12 months
d. Has had two or more exacerbations in the previous 12 months
e. Has had two or more exacerbations requiring hospitalisation in the previous 12 months
10. The following five vaccines are all recommended for patients for COPD. Which two are currently funded for patients with COPD?
a. COVID-19
b. Influenza
c. Pertussis
d. Pneumococcal (PCV13 followed by 23PPV)
e. Respiratory syncytial virus
Write down your answers to these questions. Then, to check your answers and record your score, click here.
You can use the Capture button below to record your time spent reading and your answers to the following learning reflection questions, which align with Te Whanake reflection requirements (answer three or more):
- What were the key learnings from this activity?
- How does what you learnt benefit you, or why do you appreciate the learning?
- If you apply your learning, what are the benefits or implications for others?
- Think of a situation where you could apply this learning. What would you do differently now?
- If an opportunity to apply this learning comes up in the future, what measures can be taken to ensure the learning is applied?
- Can you think of any different ways you could apply this learning?
- Are there any skills you need to develop to apply this learning effectively?
We're publishing this article as a FREE READ so it is FREE to read and EASY to share more widely. Please support us and our primary care education resources – subscribe here
1. Agusti A, Faner R, Donaldson G, et al. Chronic Airway Diseases Early Stratification (CADSET): a new ERS Clinical Research Collaboration. Eur Respir J 2019;53(3):1900217.
2. Hopkinson NS, Bush A, Allinson JP, et al. Early life exposures and the development of chronic obstructive pulmonary disease across the life course. Am J Respir Crit Care Med 2024;210(5):572–80.
3. Stolz D, Mkorombindo T, Schumann DM, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet 2022;400(10356):921–72.
4. Murgia N, Gambelunghe A. Occupational COPD–The most under-recognized occupational lung disease? Respirology 2022;27(6):399–410.
5. Landrigan PJ, Fuller R, Acosta NJR, et al. The Lancet Commission on pollution and health. Lancet 2018;391(10119):462–512.
6. Australian Commission on Safety and Quality in Health Care. Chronic Obstructive Pulmonary Disease Clinical Care Standard. October 2024.
7. Myers LC, Quint JK, Hawkins NM, et al. A research agenda to improve outcomes in patients with chronic obstructive pulmonary disease and cardiovascular disease: An official American Thoracic Society research statement. Am J Respir Crit Care Med 2024;210(6):715–29.
8. Global Initiative for Chronic Obstructive Pulmonary Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2024 Report.
9. Cushnahan A, Leaver B, Dunne B, et al. Environmental impact of pressurised metered dose inhalers versus dry powder and soft mist inhalers at a tertiary Melbourne hospital. Intern Med J 2024;54(11):1898–02.
10. Health New Zealand. Immunisation Handbook 2025, version 1.
11. Landers A, Wiseman R, Pitama S, Beckert L. Severe COPD and the transition to a palliative approach. Breathe (Sheff) 2017;13(4):310–16.
12. Maddocks M, Lovell N, Booth S, et al. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet 2017;390(10098):988–1002.
13. Williams PJ, Buttery SC, Laverty AA, Hopkinson NS. Lung disease and social justice: Chronic obstructive pulmonary disease as a manifestation of structural violence. Am J Respir Crit Care Med 2024;209(8):938–46.
14. Landers A, Pitama SG, Green SC, Beckert L. Policy, system and service design influence on healthcare inequities for people with end-of-life chronic obstructive airways disease, their support people and health professionals. BMC Health Serv Res 2024;24(1):1190.
15. Royal Australasian College of Physicians. Professional Practice Framework.
16. Soriano JB, Lumbreras S, Celli BR, Jenkins CR. I love you with all my lungs: a viewpoint on communicating effectively and positively about lung health. Eur Respir J 2024;64(1):2400919.