For older people and frail people, the long-term benefit of medicines reduces and the potential for harm from adverse effects increases. When the benefit–risk balance changes in this way, medicine review and optimisation are important to simplify the therapeutic regimen, reduce inappropriate medicines and minimise risks. In this article, pharmacist prescriber Linda Bryant uses two case studies to illustrate important considerations during medicine reviews
Prostate cancer
Prostate cancer

Prostate cancer consultations are common in primary care and there is no one approach to testing and treatment that fits all patients. Urologist Simon van Rij discusses the key considerations and options for personalised care, appropriate investigation and treatment
This How to Treat has been endorsed by the RNZCGP and has been approved for up to 1 credit for continuing professional development purposes (1 credit per learning hour). To claim your CPD credits, complete the assessment at the end of this page, then log in to your Te Whanake dashboard and record this activity under the appropriate learning category.
Nurses may also find that reading this article and reflecting on their learning can count as a professional development activity with the Nursing Council of New Zealand (up to 1 PD hour).
At the end of this course, you should be able to:
-
Explain the appropriate use of the prostate-specific antigen test in men
-
Describe the risk factors for prostate cancer
-
Discuss the options available for diagnosis and treatment of localised prostate cancer
-
Discuss the options available for staging and treatment of metastatic prostate cancer
Patients with prostate cancer are seen every day in general practice. At each stage – from the decision to investigate for cancer right through to palliative care for advancing metastatic disease – GPs see it all. And the statistics reflect this. In New Zealand, prostate cancer is the most common cancer in men and the second most common cause of cancer death. In 2018 (the latest census data), 4176 men were diagnosed with prostate cancer and 495 men died from it.
Yet, for such an important public health issue, there remains considerable controversy and numerous frequently raised questions surrounding prostate cancer:
- Don’t all men die with prostate cancer rather than from the condition?
- Does early detection confer any benefit, or does it just lead to overtreatment and side effects?
- Who needs treatment and who should avoid it?
Currently, in New Zealand, there is no recommendation for population-based screening for prostate cancer. This stance is echoed in all major urological and cancer society guidelines throughout the world.
Screening is often viewed as a “dirty word” in relation to the prostate. At an individual level, if diagnosed with stage I or II prostate cancer, survival is close to 100 per cent at five years, but if diagnosed at stage IV, the survival rate is about 50 per cent at five years. However, population screening must clearly show – for the whole population, not just the individual – a health benefit by reducing either morbidity or mortality while minimising harm and being cost-effective.
The challenge in prostate cancer is that the time from early detection to symptoms or death is, for most men, greater than 10 years. Running trials and following men for such a long time, and accounting for competing morbidity, has made the evidence difficult to interpret.
Current evidence from large, population-based, randomised controlled trials shows a reduction in prostate cancer mortality but no change in all-cause mortality with prostate cancer screening. Specifically, at 13 years, it is possible to prevent 1.3 deaths and three cases of metastatic disease for every 1000 men screened (Figure 1). This, however, comes at the expense of overdiagnosis and overtreatment for potentially 20 per cent of the men diagnosed with cancer. With further follow-up of this trial at 18 years, the number needed to screen and treat to prevent death continues to drop. This highlights the slow growth rate of prostate cancer as well as its importance with our ageing population.
In 2018, the US Preventive Services Task Force changed its grade D recommendation (no role for screening for prostate cancer) to a grade C recommendation (shared decision-making) for men aged 55 to 69. This change resulted from a reassessment of the updated evidence and an increasing awareness of the flaws and study population contamination of the large US screening trial. The task force is currently updating its recommendations again.
Current New Zealand recommendations on prostate cancer screening can be obtained from the Ministry of Health’s Prostate Cancer Management and Referral Guidance (tinyurl.com/MOH-prostate). The recommendation is for a shared decision-making process between health professional and patient. This should involve a discussion of the benefits and risks of screening.
This discussion almost exclusively occurs in primary care, with the GP responsible for trying to fit this into a standard appointment. Decision-making aids can be helpful but finding the ideal one can be difficult. Unfortunately, there is no one-size-fits-all guideline for every patient. Kupe is a tool sponsored by the Ministry of Health that attempts to provide support for this (kupe.net.nz). There are direct links on some patient management systems. Patients these days are efficient in using the internet and should be encouraged to seek out and learn from good-quality, up-to-date patient advocacy websites.
Figure 1. US Preventive Services Task Force resource for health professionals and patients
(Download the PDF here)
Ensure patients who have undergone testing are appropriately referred and/or followed up
Screening for prostate cancer in asymptomatic men is currently based on the prostate-specific antigen blood test. PSA is a liquefying enzyme found in high levels in semen. Small amounts leak into the bloodstream and can be measured. An increasing blood PSA level can be used as an indicator to investigate further for prostate cancer. There is no set PSA level that indicates a man does or does not have prostate cancer, only that risk increases as the value increases.
The PSA test is specific to the prostate gland; however, it is not specific or diagnostic for prostate cancer, and the PSA level can be elevated for other reasons, including:
- infection
- inflammation
- benign prostatic hyperplasia (BPH).
A question often asked is whether bike riding or recent ejaculation raises the PSA level, and whether a man should abstain from such activities before testing. The answer has still not been found, even with a study in 2022 looking at 101 men who cycled for one hour before testing. The evidence for an effect is weak, and it also strongly reflects both the physiological and assay variability, which can be anywhere up to 20 per cent on repeat testing. Therefore, a single test should not be relied on, but always repeated after four to eight weeks before deciding to refer.
This article refers to men with prostate cancer, but transwomen and non-binary people assigned male at birth can get prostate cancer. Little is known about its incidence or the best screening practises in this group.
Is there an ideal testing pathway?
Regardless of the controversy surrounding PSA testing, it is important to ensure patients who have undergone testing are appropriately referred and/or followed up. A number of cases have been brought before the Health and Disability Commissioner in the past, involving patients who had PSA testing in primary care with variation in follow-up.
Age-specific ranges for the PSA level are used in the current New Zealand referral guidelines to identify men who are more likely to benefit from early intervention (see table below). The age bands are often considered to be “ageist”, but it is important to remember that population-level evidence has never shown that aggressive early intervention brings benefit for men with prostate cancer who are over the age of 70. This may change as life expectancy continues to increase.
Decisions should take a patient’s physiological age, not just their chronological age, into account. Referrals for men over age 70 with an elevated PSA level who do not meet the criteria will often be considered if a discussion is made about the good health and potential longevity of the patient.
The higher reference ranges for men over 70 reflect that many men in these age groups may have prostate cancer. However, the objective is not just to diagnose cancer but to determine which patients have a high likelihood of benefiting from treatment if cancer is found.
| Age group (years) | Abnormal PSA level (ng/ml) |
|---|---|
| Men aged ≤70 | ≥4.0 |
| Men aged 71–75 | ≥10.0 |
| Men aged ≥76 | ≥20.0 |
From: Ministry of Health. Prostate Cancer Management and Referral Guidance. 2015. (CC BY 4.0)
Symptoms of benign prostatic hyperplasia
Turning our attention to the symptomatic man, BPH leading to urinary symptoms is extremely common. Unless a man has very advanced prostate cancer, it is unlikely to cause such symptoms – hence, the need to stress the importance of an initial clinical examination. Prostate cancer diagnosis in symptomatic men, therefore, is often an incidental finding as part of the workup for urinary symptoms, rather than the cause of them.
There is no evidence to show men with BPH are more at risk of developing prostate cancer.
Urgent referral guidelines
The New Zealand referral guidelines give clear pathways for the management of men with obvious symptoms of advanced prostate cancer (acute neurological signs, bone pain, renal failure) with an elevated PSA level.
For all other men who do not reach these criteria, a referral for a raised PSA level is non-urgent from a medical point of view but does raise many concerns for the man and his support network while waiting. This situation requires that the patient is appropriately counselled.
The dreaded DRE – what is its role?
What role does a digital rectal examination play in the screening process? For both men and primary care doctors, DRE is a potential deterrent to the man being checked. Therefore, in some countries, it has been removed from the guidelines to allow men to continue to be checked with a PSA test.
However, DRE remains a key clinical skill, which New Zealand primary care doctors perform regularly and competently. A meta-analysis of the literature gives conflicting findings about the benefit of DRE performed in primary care.
How often to screen
With men who do not have an elevated PSA level, the difficulty is often in determining when to check the level again. Many men have extra, unnecessary PSA testing at short intervals. The evidence supports waiting at least two to four years before the next test (yearly if there is a strong family history). In men over age 75, strongly consider not repeating testing unless new symptoms develop.
A man’s risk of prostate cancer increases with each first-degree relative with prostate cancer
With the unavoidable difficulties surrounding the PSA test, is there simply a better way of testing for prostate cancer?
Selective testing of higher-risk men
Family history – a man’s risk of prostate cancer increases with each first-degree relative with prostate cancer. For example, if a brother has had prostate cancer, the man is twice as likely to have prostate cancer compared with the general population. If both his brother and father have had prostate cancer, his risk is three times higher. It is important to be specific in questioning the patient as many family members will have, or have had, “prostate problems”.
Ethnicity – African American men have a documented higher risk of prostate cancer. There is also growing evidence to show the death rate from prostate cancer is higher in the Māori population. The causation for this is not yet known, but it may, in part, be due to a lack of early testing in Māori. Evidence shows Māori men are 50 per cent less likely to have a PSA test in primary care compared with non-Māori.
Genetics – there is increased understanding of the genetic predispositions to prostate cancer. Carriers of BRCA1 gene mutations have a 2.5-fold increased risk of developing prostate cancer, while carriers of BRCA2 mutations have an eightfold risk. Remember that such mutations can be inherited from the maternal side. There is no role for prophylactic treatment in these patient groups, but a high level of suspicion is needed.
Supplementary or alternative tests
A number of different biological tests are available but not utilised or easily available in New Zealand. None of these tests diagnose cancer; rather, they attempt to alter the pretest probability more accurately than the PSA test. The free-to-total PSA ratio is no longer used and is only available via a urologist Special Authority application.
Multiparametric MRI
Traditional prostate biopsy is, in essence, a blind biopsy of the prostate, sampling the areas most commonly associated with cancer – to find the needle in the haystack.
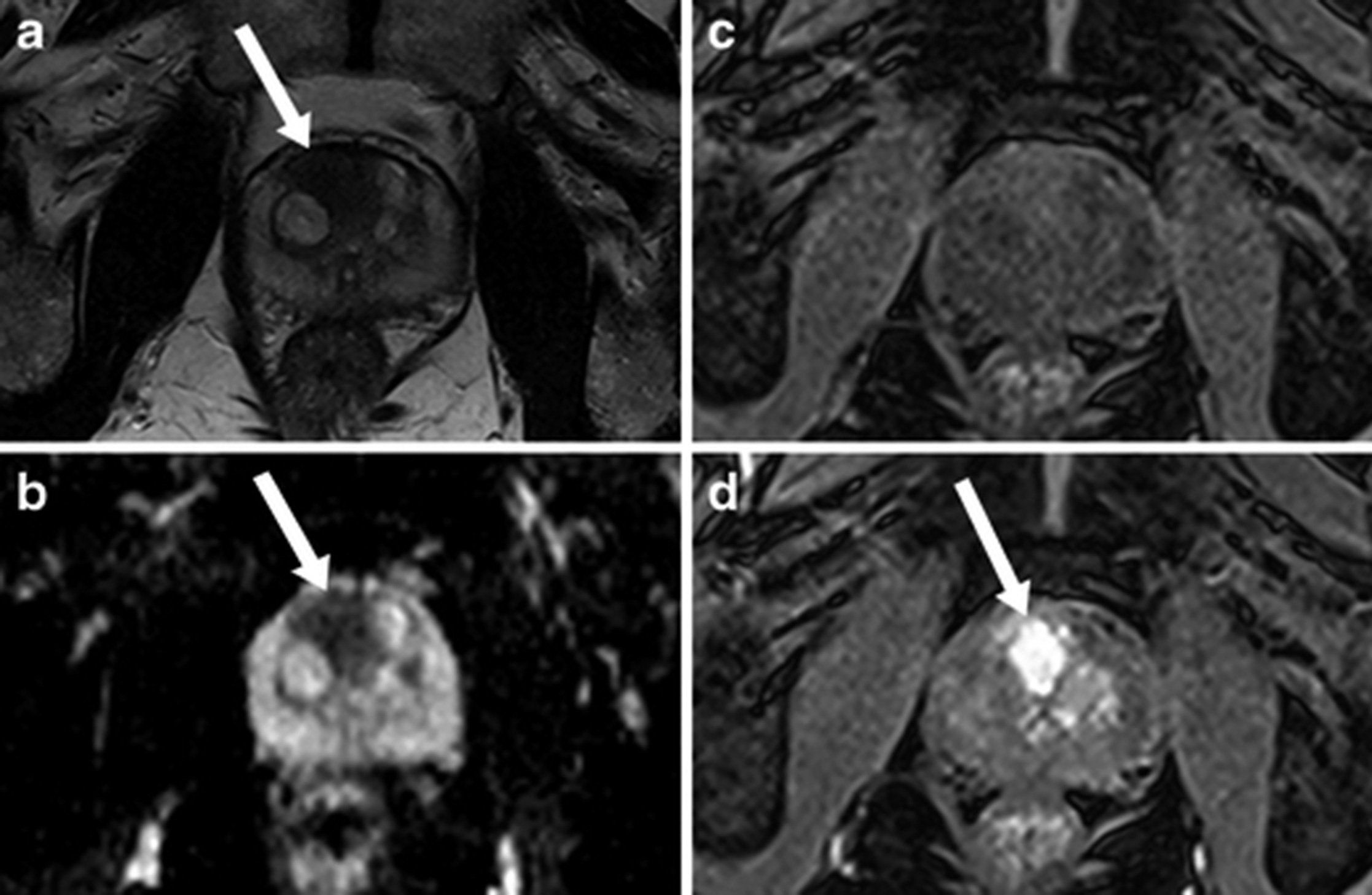
Multiparametric (multiple phase) MRI scanning of the prostate gives an accurate assessment of the gland and utilises a Likert scale to illustrate the predicted risk of prostate cancer. It also allows any areas of abnormality to be targeted if biopsy is performed (Figure 2).
There is clear evidence that MRI scanning before prostate biopsy has better rates and accuracy of cancer detection. The negative predictive value of MRI allows many men with no abnormality on MRI to avoid biopsy altogether because of their low risk.
The concerns with MRI are that it can miss certain types of prostate cancer, and many patients may still require a biopsy despite a negative MRI result. There is also the potential for a significant cost burden to the healthcare system if everyone with a suspected cancer has an MRI.
In men who have an MRI that shows no abnormality and are deemed not to require a biopsy, it is important to note that their new PSA threshold for referral for further investigation will be different from the population levels. This should be given on any discharge letters but is generally calculated using a PSA density of >0.15ng/ml/cm3 (PSA level/volume of prostate on MRI scan).
Prostate biopsy
Despite all of the above tests being available, the only way to diagnose prostate cancer is with a prostate biopsy. A referral to a urologist is made and there is further discussion of investigation and counselling to ensure the patient is well informed of their choices.
Biopsy is performed with a core biopsy needle under ultrasound guidance with a probe in the rectum. It can be performed under either local or general anaesthetic, depending on the patient and available resources.
Traditionally, the needle is passed through the rectum to sample the prostate – called a transrectal biopsy. However, with increasing rates of fluoroquinolone-resistant bacteria, there is a risk of sepsis due to faecal contamination of the prostate and urinary tract.
Transperineal prostate biopsy, with the needle passed through the perineal skin, reduces this risk of contamination and provides a potentially easier platform to target all areas of the prostate. With the increased use of MRI before biopsy, these images can be used to perform a targeted biopsy of prostate abnormalities.
Having different approaches to biopsy (both forms are available in many New Zealand public hospitals) allows it to be tailored to the patient.
- An isolated, raised PSA level (see table) in an asymptomatic man is not considered abnormal until it is repeated four to eight weeks later – if it returns to the reference range, further investigation is not required.
- Supplementary investigations are helpful to risk stratify patients, but a biopsy is required to confirm whether prostate cancer is present.
Scoring systems give important prognostic information to help patients make informed decisions about treatment. Prostate cancer diagnosed on biopsy was traditionally graded using the Gleason score. However, this system proved confusing for some patients, so in 2016, the International Society of Urological Pathology (ISUP) grading system was introduced. It gives the patient a simpler grading score: 1 = low risk of spread; 2–3 = intermediate risk; 4–5 = high risk.
Staging
A biopsy does not give information on whether or not prostate cancer has spread. Therefore, imaging is required to reveal if the cancer is localised, and this influences treatment decisions.
Men diagnosed with intermediate or low-risk prostate cancer have a very low chance of metastatic disease, so complete body staging is rarely required.
Men diagnosed with high-risk cancer have a higher risk of metastatic disease; traditionally, a bone scan or full-body CT was performed.
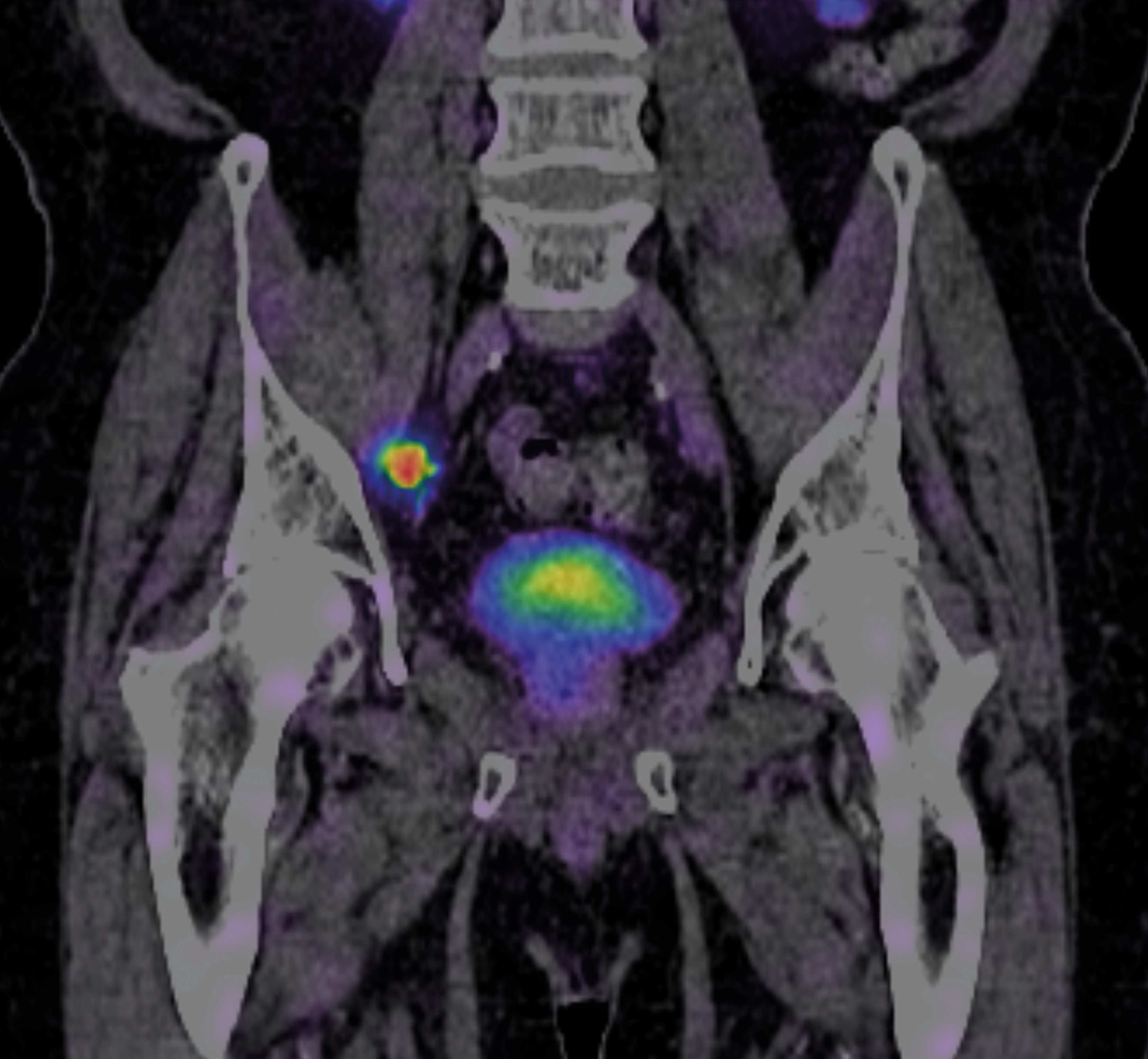
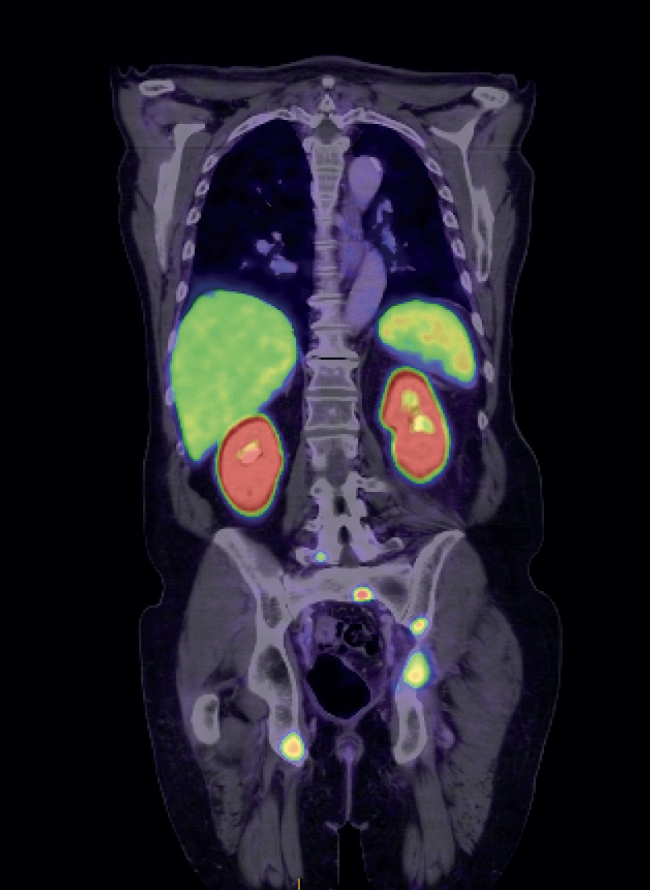
Advanced imaging with positron emission tomography (PET) allows the most accurate assessment of cancer burden (Figure 3). In 2023, prostate-specific membrane antigen (PSMA) PET was made available for all public patients who meet specific criteria. It has been a game changer in guiding the best methods of treatment for men with advanced prostate cancer and recurrent cancer.
Figure 3. PSMA PET scan of a patient with a prostate cancer metastasis (tracer uptake in a lymph node)
Alex is a 52-year-old patient. A PSA test returns a level of 2.1ng/ml, and his rectal examination is benign. Alex’s brother had been treated for prostate cancer at age 58, and his father died of prostate cancer at 73. An MRI scan is performed, which reveals an anterior lesion of the prostate, and an MRI-targeted transperineal prostate biopsy reveals ISUP grade 3 cancer (Gleason score 4+3).
Learning points
- Even with a low PSA level, a strong family history is a predictor of significant prostate cancer.
- Anterior prostate cancer is not palpable on rectal examination.
One of the most difficult decisions for men who have localised prostate cancer is which form of treatment to choose
Decisions around treatment for prostate cancer are based on the chance of the cancer causing harm within the patient’s lifetime. A general rule of thumb is that if a patient has less than a 10-year life expectancy, the benefit of early intervention will not be reached and, therefore, the risks of treatment side effects can be avoided.
However, as a doctor, predicting life expectancy is extremely difficult, as it is for clinicians to decide who should be treated. In particular, with our ageing population and increased longevity, we are now having to make this decision for patients aged 80 and older.
The following treatment options are considered for localised prostate cancer.
Active surveillance
Men diagnosed with low-risk prostate cancer (ISUP grade 1) can consider all forms of treatment, but the recommendation for all such patients is to consider active surveillance.
Active surveillance is the process of actively monitoring the cancer to detect any signs of progression. It involves regular checks, PSA level testing, MRI surveillance and further biopsy. There are different protocols for this, but it is run from within the urology departments and involves a combination of virtual and face-to-face visits.
If progression is identified, it allows the initiation of curative treatment while the cancer is still localised. By avoiding or delaying treatment, patients avoid potential side effects of surgery or radiation.
Active surveillance is safe, with 15-year follow-up data showing 95 per cent cancer-specific survival, and approximately 20–30 per cent of men requiring curative treatment within this period.
Explaining the process and reassuring men of the safety of active surveillance is key to ensuring they do not feel the need for immediate curative treatment.
Surgery: Open versus robotic
One of the most difficult decisions for men who have localised prostate cancer is which form of treatment to choose. For many other cancers, there is only one option, which avoids a decision-making process, the emotional consequences and any regret about the choice made.
Surgical removal of the prostate involves removing the prostate gland, with or without the local lymph nodes, and anastomosing the bladder to the cut urethra. Surgery can be performed as an open incision, with traditional laparoscopic technique or using minimally invasive robotic surgery. Robotic surgery is not automated surgery but uses laparoscopic, highly dexterous instruments inserted into the patient and manipulated by the surgeon at a console that is separate to the patient.
Which is the better technique – open or robotic? An Australian randomised controlled trial showed minimal difference in benefit between the two surgical techniques for cancer outcomes or long-term side effects. There was faster recovery and less blood loss with the robotic approach. The key factor is not actually the type of surgery performed but the surgeon and team who perform it.
Within New Zealand, robotic surgery is now available in the public sector, but currently only in the Auckland region due to the higher cost of this approach, although there is a push to make it more available. The trend worldwide is for all prostate surgery to be performed robotically, with 92 per cent of all surgeries in the US now performed this way.
Surgery allows for a complete histological assessment of the prostate cancer, and follow-up after surgery with the PSA blood test is a very accurate indicator of cancer recurrence. For cancer that does recur or is not completely treated at the time of surgery, radiation treatment can be provided in the future or in multimodal therapy.
The prostate is intimately related to the muscles that provide urinary continence and the nerves that facilitate erection. These can, potentially, be damaged during surgery and affect a man’s erectile and urinary function thereafter. Documenting and assessing these outcomes is important. In New Zealand, through funding from the Movember initiative, a national prostate cancer registry has been established which assesses these important patient-recorded outcome measures (prostatecancerregistry.org).
Radiation treatment
Standard radiation treatment options for clinically localised prostate cancer include external beam and/or brachytherapy, with or without androgen-suppression therapy. The aim of definitive radiotherapy for localised prostate cancer is to deliver a dose of radiation sufficient to eradicate the disease while minimising exposure of the surrounding healthy organs (bowel, bladder, penile bulb and urethra).
The multifocal nature of prostate cancer means treatment is delivered to the entire gland. New techniques allow selective intensification of the dose to the areas of higher tumour burden. Using MRI and PET scanning can further guide treatment planning.
Modern techniques can deliver radiotherapy with millimetre precision, dramatically improving outcomes and side effects. These techniques are now widely available across New Zealand.
The paradigm of radiation therapy for localised and small-volume metastatic prostate cancer has changed dramatically in recent years, and further developments are expected. In 2016, results of the CHHiP study from the UK showed that a shorter, more intense radiotherapy course delivered five days a week for four weeks (cumulative dose 60Gy) was equivalent to the conventional 7.5-week regimen (74Gy). Very recent results with extreme hypofractionation may allow treatment to condense even further, from four weeks to just 2.5 weeks in the future.
The STAMPEDE trial showed prostate radiotherapy improved survival in patients with metastatic prostate cancer compared with those who received only traditional hormonal therapy. These practice-changing findings also raise the possibility that local treatment to the primary tumour should be explored with small-volume metastatic disease in other malignant conditions.
As with surgery, over the last five years, radiation therapy has undergone unprecedented development in terms of precision, quality, efficacy and safety. Modern techniques can effectively treat localised prostate cancer and small-volume metastatic disease with improved outcomes and minimal morbidity. A large body of evidence supports the use of radiation therapy in a variety of indications for prostate cancer, reinforcing the importance of a multidisciplinary approach to treatment of all stages of this disease.
Surgery versus radiation
The patient often simply wants to know which is best, surgery or radiation therapy, but this is a difficult question to answer. This is never a decision to feel rushed into, and we can reassure patients they have time to consider all the options.
The randomised ProtecT trial from the UK looked at men with low and intermediate-risk prostate cancer. The results showed similar outcomes at 15 years between the surgery and radiation therapy groups. The decision between surgery and radiation treatment is about trade-offs between side effects and potential long-term outcomes.
To make this choice, a patient and his family must have access to accurate information and the option to discuss treatment with both a urologist and a radiation oncologist. No decision in localised prostate cancer ever needs to be rushed into, and evidence shows patient wellbeing after treatment is higher if appropriate time is taken before treatment to consider all the options.
Patient preference, resource availability and multidisciplinary clinician advice are the key factors affecting treatment choice. The few randomised trials – and a multitude of observational data – comparing surgery with radiotherapy suggest outcomes are comparable when adequate doses of radiation are delivered with contemporary techniques.
Charlie, a 76-year-old patient, presents to discuss his PSA result of 8ng/ml. He has no significant comorbidities. He discusses with his urologist the pros and cons of further investigation with a biopsy or MRI scan. After weighing up the considerations, he and his family decide not to proceed with further investigation as his other health needs are more of a priority. Charlie plans to have a PSA test done every year and, if it goes above 20ng/ml, to be referred back to his urologist.
Learning points
- For most men over 75, early identification and treatment of prostate cancer is unlikely to bring benefit. Deciding against it means a patient may never need investigation or containment/palliative management.
- PSA level of 20ng/ml in an asymptomatic man gives a 1 per cent chance of bone metastasis, which is why a level of 20–30ng/ml is often used by urologists as a signal for the referral back of men using watchful waiting.
A landmark trial showed a significant improvement in survival in men given upfront chemotherapy when they presented with symptomatic metastatic disease
Prostate cancer that has metastasised and gone unchecked can cause considerable pain, morbidity and, ultimately, death. The aim of treatment in this scenario is no longer eradication but containment of the cancer, which, in many instances, can mean survival for years rather than months.
Prostate cancer is hormonally sensitive. Blocking the supply of testosterone with male castration – either by medication or with surgery – is the mainstay of treatment for metastatic disease.
Testosterone suppression can limit prostate cancer progression for most men by an average of two to four years. However, controlling the cancer with testosterone suppression has significant side effects, for which men require ongoing management in primary care. Importantly, many of these side effects can be minimised with close follow-up. Side effects can include:
- loss of bone mineralisation requiring dual-energy x-ray absorptiometry and supplements
- metabolic changes leading to loss of muscle mass and increased risk of metabolic syndrome and cardiovascular disease
- hot flushes
- gynaecomastia
- depression
- impaired cognition.
In the last decade, some novel hormonal agents have been introduced for use in metastatic prostate cancer once traditional testosterone-suppression therapy has failed. These have increased the length of life for men and decreased cancer side effects.
Abiraterone is funded in New Zealand and is given for rises in PSA level once traditional hormonal therapy has failed. Evidence also shows that abiraterone brings benefit when given at the time of commencing traditional hormone therapy, but this is currently not funded in New Zealand. Abiraterone needs to be taken with prednisone and requires close follow-up (every fortnight on initiation) of blood pressure and liver function. Much of this can be carried out in primary care.
Traditionally, chemotherapy was seldom used in metastatic prostate cancer and was often a last resort. A landmark trial showed a significant improvement in survival in men given upfront chemotherapy when they presented with symptomatic metastatic disease. This has led to its increased use and earlier involvement of medical oncologists in the care of men with metastatic prostate cancer. They can also discuss the earlier involvement of novel hormonal agents such as abiraterone.
Further treatments for metastatic prostate cancer continue to evolve. Lutetium-177 PSMA therapy is now an established therapy for patients having progressed on all other treatments. Using the same technology as PSMA PET scans, a therapeutic rather than diagnostic radiation molecule is attached to the tracer and binds the PSMA-avid cancer tissue, providing targeted radiation. Theranostics is a fast-evolving area of treatment for prostate cancer, but it is currently not available in the public health system.
The care of men with metastatic prostate cancer is truly multidisciplinary. Survivorship is important as many men live with the condition for many years. Understanding this and minimising the side effects of both medication and cancer progression is required.
Palliative care
The perception of prostate cancer as a slow-growing disease means it is possible to forget the significant impact it causes and sometimes delay advanced care. Prostate cancer is the second most common cause of cancer death in New Zealand, and for many men in the palliative stage, it can cause significant side effects – both local (urinary obstruction, retention, haematuria, pelvic pain) and distant (bone pain, spinal nerve compression, paraneoplastic effects.
Brian is a fit and well, 61-year-old sales manager who presents with some mild urinary symptoms of urgency. He has a first PSA test, which shows a level of 17.6ng/ml, and he has an abnormal rectal examination.
A biopsy shows ISUP grade 5 prostate cancer (Gleason score 5+5). His PSMA PET scan identifies multiple bone metastases. Brian is not considered a candidate for curative treatment but can be given multiple different therapies to promote a long survival.
To begin with, Brian is given docetaxel chemotherapy by the medical oncologist, with combination hormonal therapy. The bone metastases undergo stereotactic radiotherapy provided by the radiation oncologist.
Learning points
- Advanced prostate cancer care involves a team approach from the urologist, medical oncologist and radiation oncologist.
- Metastatic prostate cancer is not an “instant death sentence”; with a combination of treatments, men can remain well for years rather than weeks.
- The difficulty with treatment is often managing the symptoms and side effects.
Douglas, an 82-year-old man, presents with worsening back pain, faecal incontinence and worsening mobility. A rectal examination reveals loss of anal tone and an obviously hard, craggy prostate. A PSA test shows a result of 752ng/ml. Doug is referred immediately to the emergency department for suspected cauda equina syndrome, which is confirmed on MRI.
Learning points
- There are few true emergencies in prostate cancer, and these are rare with most men having a PSA test at some point; however, if recognised, the patient should be referred urgently.
- A rectal examination can give the diagnosis – in this case, both from a neurological point of view and from a cancer point of view.
Details of case studies have been changed to protect patient confidentiality
Simon van Rij is a urologist and clinical lead for urology at Te Toka Tumai Auckland
This 10-question multiple-choice assessment is designed to demonstrate that the provided educational reading has been effective in allowing you to meet the learning objectives of this course. Write down your answers to these questions.
1. The evidence for prostate cancer screening and its role in New Zealand are best reflected by which three of the following statements?
a. Large randomised controlled trials show prostate cancer screening reduces all-cause mortality
b. Large randomised controlled trials show prostate cancer screening reduces prostate cancer mortality
c. New Zealand is anomalous in having no recommendation for population-based prostate cancer screening
d. Prostate cancer screening results in cases of overdiagnosis and potential overtreatment
e. The New Zealand recommendation regarding prostate cancer screening is for shared decision-making between patient and doctor
2. The purpose of the prostate-specific antigen test before treatment is to achieve which two of the following?
a. To diagnose prostate cancer
b. To exclude benign prostatic hyperplasia
c. To identify patients at higher risk of localised prostate cancer who can consider further investigation
d. At higher levels, to identify those more at risk of metastatic prostate cancer
3. When giving a patient a PSA test result that is within the reference range for their age, you should potentially inform him of which of the following? (Three correct answers.)
a. He does not require immediate further investigation
b. He should have another PSA test in one year
c. He should have another PSA test in two to four years
d. If he has a strong family history of prostate cancer, he should consider having yearly PSA tests
e. If he is older than 75, he should consider having yearly PSA tests
4. When giving an asymptomatic patient an initial test result showing a slightly raised PSA level, you should also do which two of the following?
a. Arrange for a repeat PSA test in four to eight weeks
b. Explain that cancer is not the only cause for a raised PSA level
c. Explain that the test result is diagnostic for prostate cancer
d. Recommend making a urology appointment for further investigation
5. What raises the risk of prostate cancer the most?
a. Carrying a BRCA1 mutation
b. Carrying a BRCA2 mutation
c. Having a brother with prostate cancer
d. Having a brother and father with prostate cancer
6. Biopsy is the only way to diagnose prostate cancer. Which two statements regarding prostate biopsy are correct?
a. A positive biopsy result will also give an indication of whether the cancer has spread
b. Biopsy can be performed under either local or general anaesthetic
c. MRI prior to biopsy has no advantage over ultrasound-guided biopsy alone
d. The transperineal route reduces the risk of faecal contamination and sepsis
e. The transrectal route provides the best access to all areas of the prostate
7. For a man recently diagnosed with low-risk (ISUP grade 1) prostate cancer, which two pieces of advice are appropriate?
a. He can consider all forms of treatment, including active surveillance, surgery and radiotherapy
b. He should make his decision about whether to have curative treatment as quickly as possible while the cancer is still low risk
c. He should strongly consider active surveillance
d. If he chooses active surveillance, he should still think about curative treatment as he will most likely need it within the next five to 10 years
8. When the decision to commence curative treatment for localised prostate cancer has been made, what is the best approach for most patients?
a. Brachytherapy
b. External beam radiotherapy
c. Open surgery
d. Robotic surgery
e. There is no clear best choice
9. For men diagnosed with high-risk prostate cancer, what is the most accurate way to assess their cancer burden?
a. Bone scan
b. Full-body CT scan
c. MRI scan
d. PSMA PET scan
e. Ultrasound scan
10. For metastatic prostate cancer, blocking the supply of testosterone is the mainstay of treatment. Which two statements regarding this form of treatment are correct?
a. Early involvement of novel hormonal agents and/or chemotherapy (when traditional hormone therapy is commenced rather than after it has failed) is beneficial
b. Once testosterone-suppression therapy has failed, there are no further options to extend life
c. Testosterone suppression limits cancer progression by up to one year on average
d. The side effects of testosterone suppression can be minimised in primary care
Write down your answers to these questions. Then, to check your answers and record your score, click here.
You can use the Capture button below to record your time spent reading and your answers to the following learning reflection questions, which align with Te Whanake reflection requirements (answer three or more):
- What were the key learnings from this activity?
- How does what you learnt benefit you, or why do you appreciate the learning?
- If you apply your learning, what are the benefits or implications for others?
- Think of a situation where you could apply this learning. What would you do differently now?
- If an opportunity to apply this learning comes up in the future, what measures can be taken to ensure the learning is applied?
- Can you think of any different ways you could apply this learning?
- Are there any skills you need to develop to apply this learning effectively?
We're publishing this article as a FREE READ so it is FREE to read and EASY to share more widely. Please support us and our primary care education resources – subscribe here